The amendment that modifies Regulations (EU) 2017/745 and (EU) 2017/746 with respect to transitional provisions for certain medical devices (MD) and certain in vitro diagnostic medical devices (IVD) has just been adopted by the European Parliament.
This amendment was initiated by a common will between the European Parliament, Member States and stakeholders (health professionals, patients, academia, scientific bodies, industry and notified bodies). It responds to the unanimous observation that there is a risk of a shortage of medical devices that would jeopardize patients safety and hinder innovation in the EU health sector. This situation is exacerbated by the effects of the COVID-19 pandemic on clinical investigations, on-site audits, and global supply chains.
The amendment provides for the extension of the transition period by extending the validity of certificates issued under the directives.
This approach aims to strike a balance between the available capacity of notified bodies and the level of preparedness of manufacturers, while ensuring a high level of public health protection.
Nevertheless, it is important to emphasize that the provisions made in this text do not prevent you from starting now certain actions related to your transition to the new regulations. Indeed, this extension should not be considered as additional time at the risk of causing a new bottleneck at the next deadlines.
To help you understand the ins and outs of this new text, GMED has analyzed the different key paragraphs and the impact for manufacturers and notified bodies.
Provisions introduced
The amendment modifies the transitional provisions and addresses only those devices for which the intervention of a notified body under regulation is necessary. It allows manufacturers who have engaged or will engage before 26/05/2024 in a conformity assessment process under Regulation (EU) 2017/745 (MDR) for their medical devices, to continue placing their medical devices on the market under certain conditions (“legacy devices”). Europe’s Institutions ensure through these provisions that only devices that are intended to be compliant or to be substituted by devices compliant with Regulation (EU) 2017/745 are placed on the market after 26/05/2024. The text enters into force on the day of its publication in the Official Journal of the European Union.
The amendment allows manufacturers to reintroduce devices with expired MDD or AIMDD certificates when a formal application for MDR certification has been filed and the certification contract with a MDR-NB has been signed prior to the expiry of the said certificate.
Modification of the calendar
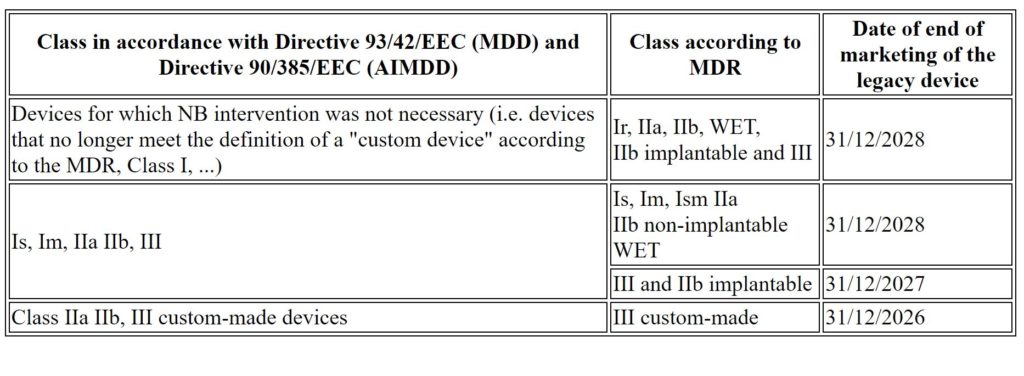
The schedule is defined as follows, based on the MDR classification of devices:
Removal of the « sell-off” deadline
The amendment removes the date after which devices can no longer be made available (“sell-off” deadline). Legacy devices can therefore continue to be made available on the market and put into service after 26/05/2025. This removal applies unconditionally: devices that will not be brought into compliance with the MD regulation are also beneficiaries.
Furthermore, this provision also applies to in vitro diagnostic medical devices placed on the market under Article 110.3 of Regulation (EU) 2017/746.
Certificate management
Nothing in the amendment requires the reissuance or modification of certificates issued under the Directives. Certificates are deemed to be de facto extended, provided only that they have not been withdrawn.
Conditions to benefit from the transitional provisions
The extension of the period during which the devices can be placed on the market is subject to conditions.
To benefit from the new provisions, the manufacturer must:
- Have implemented and maintain a Quality Management System that complies with the requirements of Article 10, paragraph 9 of the MDR before 26/05/2024;
- before May 26th, 2024, have lodged its formal application for certification in application of the MDR for each device with a Notified Body designated against the MDR and have signed a contract with the latter which covers the concerned devices before September 26th, 2024.
In addition, the devices:
- Must remain in compliance with the requirements of the MD or AIMD Directive applicable to them;
- Must not undergo any significant change in the design or intended purpose;
- Must not present an unacceptable risk to the health or safety of patients, users or other persons, or to other aspects of the protection of public health;
Devices that have been withdrawn from the MDD/AIMDD certification scope cannot benefit from the new transitional provisions.
Note: For custom implantable Class III devices, the manufacturer shall:
- Ensure compliance of its MD with all requirements of Regulation (EU) 2017/745 applicable to custom-made devices, with the exception of obtaining an EU certificate of conformity issued under Regulation;
- Have filed its formal application for certification with a Notified Body designated under the MDR before 26/05/2024;
- Have signed a contract with a Notified Body designated under the MDR before 26/09/2024.
GMED reiterates that it is the responsibility of the manufacturer to ensure that each legacy device placed on the market complies with the provisions of section 120.3.
It should be noted that the submission of a formal application and the signing of a contract with a Conformity Assessment Body in the process of being designated under the Regulation does not allow manufacturers to benefit from the transitional provisions.
Surveillance of legacy devices
Prior to September 26, 2024, surveillance of legacy devices is the responsibility of the Notified Body that issued the certificate under the applicable Directive, unless the manufacturer has agreed with the MDR NB with whom it has a contract under the MDR that the NB will perform the surveillance.
As of September 26, 2024, the NB with which the manufacturer has signed a MDR contract is responsible for the surveillance of legacy devices covered by the application for certification and the MDR contract.
In both cases, the arrangements for the transfer of surveillance should be defined in a contract between the manufacturer and the NB with which the manufacturer has applied for MDR certification, and where possible with the NB that issued the certificate under the Directive.
If your directive certificate expired before the amendment came into force and
- you have signed a contract in application of the MDR before its expiry date, or
- if you have an authorization to continue to place the devices on the market issued under article 59 or 97 of the MDR
we invite you to contact GMED so that a contractual terms and conditions for the surveillance of your legacy devices can be established.
Conclusion
As we have seen, the changes made aim to maintain the availability on the European market of a wide range of medical devices while ensuring the gradual transition to the new framework.
However, we would like to remind you that, despite this postponement, we strongly encourage manufacturers to continue their transition efforts in order to avoid the risk of an imbalance between the available capacity of the notified bodies and the demands for certification at the next deadlines.
GMED, as a Notified Body, is committed to the medical device industry in the effort to certify legacy devices. To do so, we invite you to contact the GMED teams now to learn about the solutions implemented for the transitional provisions.
- For Europe enquiries, contact GMED SAS: sales@lne-gmed.com
- For America or International enquiries, contact GMED North America: request@lne-gmed.com
share
Events
Find an answer to the challenges you are facing in one of our upcoming events: trainings, webinars, forums...
